Blog dealing with TA (Technical Analysis) and charts of the Biotech sector, following companies during the run-up till the Catalyst date
Wednesday, November 30, 2011
$SPX The SP-500 bouncing from the Fibo 61.8 correction
Lots of pressure was in the markets, we had a consecutive 7 red days, some traders start looking again for the double dip, some get bearish and went short or sold and step away from the markets..
The Fibonacci correction is a healthy correction for every security or the markets after a long run up, we can see that the Fibo 61.8 used as a support, holding the price around 1,158, creating a reversal candle stick (Inverted Hammer) , and complete the reversal with a long green candle (Marubozu candle stick) to give the sign for the new uptrend.
Look at the indicators too, the Stochastics & the RSI was deep in the oversold zone, both changed direction to support the new uptrend breaking up the 50MA , now looking for the 200MA as a target price.
Today the price will find a resistance at the long period uptrend line (Green line) around 1,240 and after that will attack the 1,258 resistance line and very close to the 200MA.
If the price fail to breakup the 1240 point, we can see a technical correction for the last move, and we can see the price touching again the 1200 point, just before keep the real uptrend.
Breaking down the 1200 point will change the direction and I have to recheck the charts again
Have a nice trading week
Monday, October 17, 2011
$YMI Trading in a falling wedge
$YMI trading in a falling wedge, we can see the volatility squeeze and consolidation through the Bollinger bands that get horizontal and very close to each other, the breakout of the upper band and the downtrend line (cyan) will lead for a wild breakout and uptrend toward the weekly resistance point @ $2.55 & the 200MA.
Meanwhile the price trading side-way and have to deal with the 50MA too, we might see recheck of the lower band before the breakout as the Stochastic start turning down from the overbought zone and should clear out, in the other side we can see a positive divergence in the RSI & MACD.
For the guys that already holding, keep holding as the price will start moving as we get close to Dec 2011 where the company expects to report final efficacy data from P3 adult brain cancer (glioma) trial till year end, and is enrolling patients in ongoing P2B/3 trial for pancreatic cancer
The second catalyst is CYT387 (a JAK1 / JAK2 Kinase Inhibitor) YMI Expects to report data from expanded group of patients (166 enrolled) in ongoing P1/2 trial for patients w/ myelofibrosis in DEC 2011 (expected at ASH mtg Dec 10-13), on 9/28/11 enrolled first patients in P2 trial to evaluate twice-daily (BID) dosing, results expected 4Q12
For the guys that it's only in the watching list, Breaking out the upper falling wedge line with a large volume and closing above will be a buying point.
Disclosure: no position yet.
Thursday, October 13, 2011
$AVNR Breaking out ascending triangle
$AVNR Breaking out ascending triangle 3 days ago with large volume, it just started day earlier by breaking out the 50MA too...after the breaking out, the price usually testing back the line that used as a resistance line and supposed to use as a support this time around $3.09
This retrace created a "Bull Flag" , breaking out this flag will gave us a long run up, as long as the flag pole, but braking down the $3.09 point might start a technical correction for the last uptrend between $3.00 to $2.80 as Fibonacci levels.
Daily Chart
Looking at the intraday chart 15min we can see the creation of the bull flag...and the volume contraction with a positive momentum
Start a position @ $3.10 with a close stop lose
This retrace created a "Bull Flag" , breaking out this flag will gave us a long run up, as long as the flag pole, but braking down the $3.09 point might start a technical correction for the last uptrend between $3.00 to $2.80 as Fibonacci levels.
Daily Chart
Looking at the intraday chart 15min we can see the creation of the bull flag...and the volume contraction with a positive momentum
Start a position @ $3.10 with a close stop lose
Thursday, October 6, 2011
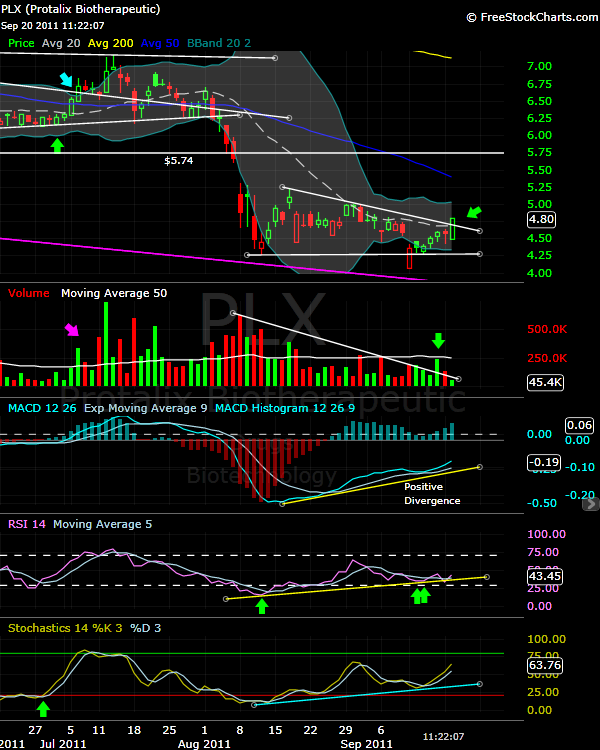
$PLX : Volatility Squeeze, looking for the break out
$PLX Technically looks very interesting, the Bollinger bands became horizental and very close to each one, (Volatility Squeeze) and we can see a very clear positive divergence in both the MACD and the RSI, beside the expansion of the volume during the up days and contraction in the down days, give us the clue of accumulation.
Protalix have a several catalyst, First, the PDUFA date on 02/01/2012, but have major catalyst during this period, as the company have to reply to the EMA (Europe) 120 day question and will be submitted during October as the IR told me last week, the management took a long time to reply to EMA 120 day question from April, I think it was smart and safer move to synchronize EMA data with FDA data.
After PLX submits the 120 day questions – EMA has 2 months to review and then comes back to PLX with a second set of questions (called 180 day questions as it's 180 days from filling). Protalix then responds and should hear shortly after that on a recommendation by CHMP.
The Committee for Medicinal Products for Human Use (CHMP) is responsible for preparing the Agency's opinions on all questions concerning medicines for human use.
Notice of formal approval would then follow the CHMP recommendation (assuming positive). and can be done during Dec 2011 or early Jan 2012.
Third catalyst is the Brazilian approval that could happened at any time (very close).
Fourth Catalyst is the Investigational New Drug (IND) Application for phase I/II clinical trial in Fabry disease will be submitted before the end of the year as noted by the CFO Mr. Yossi Maimon at Jefferies & Co. Global Healthcare Conference-UK, held on September 27, 2011, he mentioned that the FDA encourage PLX to submit an IND for phase I/II directly in humans...it's bio better product.
For the safest trade, start position after breaking out the upper Bollinger band.
Disclosure: Long $PLX
Daily Chart
Protalix have a several catalyst, First, the PDUFA date on 02/01/2012, but have major catalyst during this period, as the company have to reply to the EMA (Europe) 120 day question and will be submitted during October as the IR told me last week, the management took a long time to reply to EMA 120 day question from April, I think it was smart and safer move to synchronize EMA data with FDA data.
After PLX submits the 120 day questions – EMA has 2 months to review and then comes back to PLX with a second set of questions (called 180 day questions as it's 180 days from filling). Protalix then responds and should hear shortly after that on a recommendation by CHMP.
The Committee for Medicinal Products for Human Use (CHMP) is responsible for preparing the Agency's opinions on all questions concerning medicines for human use.
Notice of formal approval would then follow the CHMP recommendation (assuming positive). and can be done during Dec 2011 or early Jan 2012.
Third catalyst is the Brazilian approval that could happened at any time (very close).
Fourth Catalyst is the Investigational New Drug (IND) Application for phase I/II clinical trial in Fabry disease will be submitted before the end of the year as noted by the CFO Mr. Yossi Maimon at Jefferies & Co. Global Healthcare Conference-UK, held on September 27, 2011, he mentioned that the FDA encourage PLX to submit an IND for phase I/II directly in humans...it's bio better product.
For the safest trade, start position after breaking out the upper Bollinger band.
Disclosure: Long $PLX
Daily Chart
Monday, October 3, 2011
$TZA & $TNA holders are fighting for Direction..$IMW is the leader
$IWM have a "Descending Triangle", Lower highs and lower lows...
Breaking down the support line will be a huge short for the Russel 2000 Index...TZA & TNA holders watch it
In the other side, the RSI & MACD with a positive divergence!!!
Who is gonna win the battle
Breaking down the support line will be a huge short for the Russel 2000 Index...TZA & TNA holders watch it
In the other side, the RSI & MACD with a positive divergence!!!
Who is gonna win the battle
Tuesday, September 27, 2011
$CBRX: Trading within horizontal channel
UPDATE OCT. 15
The price breakout the channel with a long candle and large volume
The price reach the second target as it touched the resistance line @ $2.71 breakout but close below @ $2.70....
It was a very nice runup as written in the Technical Analysis books....
The momentum still very strong, the price might get some correction next week, it will be a nice to load more shares if it retreat to test back the upper channel line around $2.48
Enjoy the ride
Joe Gantoss
click the chart to enlarge
$CBRX Columbia Laboratories, Inc. - PDUFA date of February 26, 2012 for PROCHIEVE for the reduction of risk of preterm birth in women with short uterine cervical length in the mid-trimester of pregnancy.
The shares price dropped hard with the markets, but the pressure started after the NDA acceptance by the FDA as regular review 10 months and not a priority review as Columbia has voluntarily withdrawn its request for priority review.
We can see at the daily chart that it start trading in a horizontal channel between $1.95 & $2.50 , also the Bollinger bands get closer, tight & horizontal too while the RSI & the MACD are in a positive divergence and the Stochastics prepare to leave the oversold area and start a new move...
We saw yesterday the price bouncing back from the support line creating a "Bullish Hammer" at a support zone...that's a good sign as the buyers are coming back and buy the dip.
Start a Position @ $2.00 looking to recheck the upper channel line, with stop loss few points below the support line @1.95
Doubled the position @ $1.95
It might be a good start position as a the catalyst of $CBRX is 4 month away
PDUFA date02/26
Disclosure: Long $CBRX
Daily Chart
The price breakout the channel with a long candle and large volume
The price reach the second target as it touched the resistance line @ $2.71 breakout but close below @ $2.70....
It was a very nice runup as written in the Technical Analysis books....
The momentum still very strong, the price might get some correction next week, it will be a nice to load more shares if it retreat to test back the upper channel line around $2.48
Enjoy the ride
Joe Gantoss
click the chart to enlarge
$CBRX Columbia Laboratories, Inc. - PDUFA date of February 26, 2012 for PROCHIEVE for the reduction of risk of preterm birth in women with short uterine cervical length in the mid-trimester of pregnancy.
The shares price dropped hard with the markets, but the pressure started after the NDA acceptance by the FDA as regular review 10 months and not a priority review as Columbia has voluntarily withdrawn its request for priority review.
We can see at the daily chart that it start trading in a horizontal channel between $1.95 & $2.50 , also the Bollinger bands get closer, tight & horizontal too while the RSI & the MACD are in a positive divergence and the Stochastics prepare to leave the oversold area and start a new move...
We saw yesterday the price bouncing back from the support line creating a "Bullish Hammer" at a support zone...that's a good sign as the buyers are coming back and buy the dip.
Start a Position @ $2.00 looking to recheck the upper channel line, with stop loss few points below the support line @1.95
Doubled the position @ $1.95
It might be a good start position as a the catalyst of $CBRX is 4 month away
PDUFA date02/26
Disclosure: Long $CBRX
Daily Chart
Friday, September 23, 2011
$CLSN: Just around an important support point
$CLSN get hammered as the markets did the same this week...
Just a reminder from my last TA :
As we are in a bearish markets, any large decline in the markets might put pressure at the price of CLSN too, so in the other side we have to watch the support line @ $3.50 and the 50MA...sliding below those points would cancel the bull pennant pattern.
The price breakdown those support points starting with the $3.50 then the 50MA and the well known $3.10 point...we can see the price just sliding down and touching the downtrend line (brown line) all the way down.
The new support is the 200MA that used several times in the past as a real support, we can see the price action around the 200MA bouncing up from it...Today we see a positive day with a green and bullish engulfing pattern, we can see the RSI just turning up, but need to breakup the 30 line...the same with the stochastics at the oversold zone...If the 200MA didn't hold so we can see the price heading to the next strong support @ $2.71 ...
If the problems with Greece and the European union have some positive news, so we can see the price running up to test back the $3.10 point...
keep watching closely...
Disclosure : Long $CLSN
daily Chart
Just a reminder from my last TA :
As we are in a bearish markets, any large decline in the markets might put pressure at the price of CLSN too, so in the other side we have to watch the support line @ $3.50 and the 50MA...sliding below those points would cancel the bull pennant pattern.
The price breakdown those support points starting with the $3.50 then the 50MA and the well known $3.10 point...we can see the price just sliding down and touching the downtrend line (brown line) all the way down.
The new support is the 200MA that used several times in the past as a real support, we can see the price action around the 200MA bouncing up from it...Today we see a positive day with a green and bullish engulfing pattern, we can see the RSI just turning up, but need to breakup the 30 line...the same with the stochastics at the oversold zone...If the 200MA didn't hold so we can see the price heading to the next strong support @ $2.71 ...
If the problems with Greece and the European union have some positive news, so we can see the price running up to test back the $3.10 point...
keep watching closely...
Disclosure : Long $CLSN
daily Chart
Tuesday, September 20, 2011
$AMRN: Very close for the next move
We can see the price consolidation during the last month, the price found a base and support @ the horizontal support line $9.66$ bouncing from the support last month and creating an uptrend wedge (cyan lines).
Now the price have a big resistance and trying to breakup the downtrend line (huge downtrend channel) that using as a resistance line (white) since 05/31 @ the high $19.87
The RSI and the MACD are in a positive divergence too, convincing me that the breakup is very close.
Disclosure: Long $AMRN
Daily Chart
Now the price have a big resistance and trying to breakup the downtrend line (huge downtrend channel) that using as a resistance line (white) since 05/31 @ the high $19.87
The RSI and the MACD are in a positive divergence too, convincing me that the breakup is very close.
Disclosure: Long $AMRN
Daily Chart
$PLX: Breaking up the wedge might give a real uptrend
$PLX, we can see the price breaking up the upper trend line of the wedge, but we have to wait till the markets closing to see if the price could close above the line, and at least close above $4.71 point, that will be a bullish sign.
The indicators was bullish during the last trading month, we can see the MACD & the RSI trending up during the wedge formation and we can see the volume decrease, we call it a "price consolidation", but we need to see increasing volume today and the coming days to insure the breakout.
If we don't see a widening in the volume, so I think that we might have a technical correction for the last trend.
The catalyst for the last price action was the letter that Genzyme sent to the health provider indicating the new shortage of the drug supply for the Gaucher patients, this will be a good reason to push the FDA for approval a third supplier for the Gaucher drug, that's $PLX favor
During this shortage, Prptalix will deal with the European & Brazilian approval too in Q4, as my calculation the approval decision in Europe will come during November this year, and the European markets will be targeted with $PFE the worldwide marketing partner (excluding Israel) and can compete with Genzyme & Shire with a aggressive prices as the coast of the production in $PLX technology is significantly cheaper than the both competitors.
keep watching closely..
Disclosure: Long PLX
Daily Chart
The indicators was bullish during the last trading month, we can see the MACD & the RSI trending up during the wedge formation and we can see the volume decrease, we call it a "price consolidation", but we need to see increasing volume today and the coming days to insure the breakout.
If we don't see a widening in the volume, so I think that we might have a technical correction for the last trend.
The catalyst for the last price action was the letter that Genzyme sent to the health provider indicating the new shortage of the drug supply for the Gaucher patients, this will be a good reason to push the FDA for approval a third supplier for the Gaucher drug, that's $PLX favor
During this shortage, Prptalix will deal with the European & Brazilian approval too in Q4, as my calculation the approval decision in Europe will come during November this year, and the European markets will be targeted with $PFE the worldwide marketing partner (excluding Israel) and can compete with Genzyme & Shire with a aggressive prices as the coast of the production in $PLX technology is significantly cheaper than the both competitors.
keep watching closely..
Disclosure: Long PLX
Daily Chart
Friday, September 16, 2011
$PLX: is the new shotage in Genzyme will benefit Protalix
Genzyme sent a letter about short supply
Genzyme Sanofi company with it;s main orphan drug, sent again a letter about its short supply of Cerezyme that used as a drug to treat the Gauche patients again.
In a letter to health care providers sent on Sep 13, 2011, Genzyme said adult patients would probably receive only one dose a month instead of the usual two, from October through January.
The drug which costs around $250,000 a year, treats Gaucher disease, a rare inherited enzyme deficiency.
In June 2009 Genzyme was forced to temporarily close its Boston's factory, because of viral contamination. It did not restore full supplies of Cerezyme until January of this year, much later than it initially expected.
In a letter to health care providers sent on Sep 13, 2011, Genzyme said adult patients would probably receive only one dose a month instead of the usual two, from October through January.
- We would like to provide an update regarding our expectations for supply of Cerezyme in the U.S. A temporary decrease in Cerezyme yields, combined with changes to our product release processes and procedures that lengthen the overall time it takes to release Cerezyme require us to change our supply projections for the next four months starting October, 2011.
Changes to Cerezyme availability will be felt globally. Delays in shipments will likely affect patients and some adjustment to individual treatment plans may be necessary.
The drug which costs around $250,000 a year, treats Gaucher disease, a rare inherited enzyme deficiency.
In June 2009 Genzyme was forced to temporarily close its Boston's factory, because of viral contamination. It did not restore full supplies of Cerezyme until January of this year, much later than it initially expected.
The main competitor for the Gauche drug supply is Shire’s Vpriv that approved by the FDA last year but they are limited with a supply to 1000 patients, while there is 5500 patients in the world that treated with Gauche drugs from Genzyme, Shire, and rest with Protlaix (PLX) drug Uplyso taliglucerase alfa that still waiting for the FDA approval .
I think that this shortage will encourage the FDA to approve PLX, as this orphan drug need more supply to rely on as this decease is a life threatening, and the FDA knows that those shortage with Cerezyme might be for a longer periods.
- One possible beneficiary is Protalix BioTherapeutics, according to Wells Fargo Securities analyst Brian Abrahams. In an investor note, he writes that with the Cerezyme shortage “shortage occurring in the months ahead of the February PDUFA date for PLX taliglucerase, this should highlight to the FDA the need for an additional enzyme supplier and perhaps increase the likelihood of approval.”
Many believed that Taliglucerase Alfa had a shot on obtaining FDA approval (one prime example was Oppenheimer’s equity research on PLX), but on February 25, 2011, the FDA issued a Complete Response Letter (CRL) regarding the company’s NDA for Taliglucerase Alfa. A CRL is issued by the FDA's Center for Drug Evaluation and Research when the review of a file is completed and questions remain that precludes the approval of the NDA in its current form. The main questions raised by the FDA regarding the NDA related to the clinical and CMC sections. In the clinical section of the CRL, the FDA requested additional data from the ongoing switchover trial and the long-term extension trial. At the time the NDA was submitted, full data from these trials were not available. In the CMC section of the CRL, the FDA requested information regarding testing specifications and assay validation. The FDA did not however request additional clinical studies in the CRL.
One thing to note is that the marketing applications filed in the European Union, Brazil and Australia include certain data now being requested by the FDA in the CRL . These applications (the marketing applications) were submitted after filing the NDA, during which Protalix had collected additional data from these ongoing trials.
Form the Technical side, we can see that $PLX is trading in a "Positive Divergence" in the past few weeks, as the price drop but the momentum is gaining all the time, just looking @ the MACD & RSI we can see the positive divergence very clear, and the volume is decreasing in the down days...that's show a price consolidation that will lead for a new uptrend in the near future
Don't forget that beside the main catalyst of the FDA approval as the PDUFA date on Feb 01, 2012...but meanwhile, $PLX have 2 other important catalyst set for Q4, the EU & Brazilian approval for Taligluceraee Alfa that could take the price for a new run-up even before the FDA approval.
Look at the channel breakup for a new trend, the price should breakup the 20MA too then the 50MA waiting around the $5.40 point as a resistance too...
Disclosure: Long $PLX
Daily Chart
Sunday, September 11, 2011
$CLSN: Price consolidation
As I wrote on my last TA on Aug 31st CLSN had lots resistance points.
The price did retreat and check the $3.50 and find a good support with the 50MA (Blue Line) too, bouncing back and close above the $3.50 resistance line...we can see a price consolidation creating a Bullish Pennant pattern, while the $SPX found in a bearish mode....That's a bullish sign for $CLSN, and we can see that the volume decreased within the pennant sign for the consolidation.
Pennants are short-term continuation patterns that mark a small consolidation before the previous move resumes. These patterns are usually preceded by a sharp advance or decline with heavy volume, and mark a mid-point of the move.
Breaking up the upper line (white) will give us a technically move that we can calculate it as the flagpole length of about $0.75 that might take the price after the breakup to $4.50 at least, but we have to watch the volume...Volume should be heavy during the breakup, An expansion of volume on the resistance break lends credence to the validity of the formation and the likelihood of continuation.
As we are in a bearish markets, any large decline in the markets might put pressure at the price of CLSN too, so in the other side we have to watch the support line @ $3.50 and the 50MA...sliding below those points would cancel the bull pennant pattern.
Disclosure: Long CLSN
Daily Chart
This area is very complicated, full of support & resistance points,
the main resistance now is the uptrend line that used as a support for a
long period, and these days change his role and might used as a
resistance line (the green line) we can see that the price break out the
line, but retreat and close below the line. We might see a correction and recheck the $3.50 line that supposed to use as a support line this time, one more point is the price went out from the upper Bollinger Band, and might get some pressure to get back inside the bands, in the other side, breaking out the uptrend line will find another resistance point @ $3.87
Pennants are short-term continuation patterns that mark a small consolidation before the previous move resumes. These patterns are usually preceded by a sharp advance or decline with heavy volume, and mark a mid-point of the move.
Breaking up the upper line (white) will give us a technically move that we can calculate it as the flagpole length of about $0.75 that might take the price after the breakup to $4.50 at least, but we have to watch the volume...Volume should be heavy during the breakup, An expansion of volume on the resistance break lends credence to the validity of the formation and the likelihood of continuation.
As we are in a bearish markets, any large decline in the markets might put pressure at the price of CLSN too, so in the other side we have to watch the support line @ $3.50 and the 50MA...sliding below those points would cancel the bull pennant pattern.
Disclosure: Long CLSN
Daily Chart
Friday, September 9, 2011
$TZA: The Small Cap indicator - Inverse H&S
The markets are trading within a Flag pattern in the past month, most of the indices are in a short term uptrend channel , most of the technician believes it's mostly a "Bear Flag"... breaking down the lower line will trigger a big downtrend.
$TZA is a Small Cap Bear 3X and show us the strength of the bears.
If we look at the 1 hour chart, we can see a very nice Inverse Head & Shoulders bearish pattern..
For the traders that are not using a Technical Analysis. here is the definition of Inverse H&S:
A chart pattern used in technical analysis to predict the reversal of a current downtrend. This pattern is identified when the price action of a security meets the following characteristics:
1. The price falls to a trough and then rises.
2. The price falls below the former trough and then rises again.
3. Finally, the price falls again, but not as far as the second trough.
Once the final trough is made, the price heads upward toward the resistance found near the top of the previous troughs. Investors typically enter into a long position when the price rises above the resistance of the neckline. The first and third trough are considered shoulders, and the second peak forms the head
If we look at the chart below, we can see the Head & Shoulders with the Blue lines...if the price of $TZA breakup the Neck-Line around $52 with a large volume we can see the Brown Line Scenario for the uptrend, but if the line used as a resistance, we will have the Green Line Scenario testing a new lows and canceling the H&S pattern....
Next week is an important week for the markets, decisions should been taken about the trend...
Have a nice weekend
1hour chart
Thursday, September 8, 2011
$BPAX Technical Analysis: UPDTAE - Trading in an ascending triangle
In the past 4 weeks, we can see a price consolidation creating an Ascending Triangle ,we can see on the daily chart bellow higher lows, as the traders are paying more for the same shares.
The $2.50 horizontal line use as a support / resistance since 2009, and this days we can see a fight around this point too.
The indicators showing a strength as both the MACD & the RSI-14 with a Positive Divergence that gave me the confidence that the direction is upward, is might trade inside the triangle, but breaking up the upper line (white line) will give us a big move upward and we can calculate it as the side of the triangle that will give us a $0.80 at least...so the technical target is $3.60 at least....
Today. the price breakup the $2.50, but we need to see the price after the market closing, the next point is breaking up the upper line around $2.80.
Update....
Just looking at the chart we can see that the price reach the target and touch the upper trend line of the triangle...with a large volume...that's give us more confidence that the break up is very close...
We might see a profit taking @ this hights..as lots of technicians put a sell order just points under a resistance points...but, after a small correction we will have the uptrend even faster and will take the price to the next resistance point @ $3.20
I'm still long and waiting for the next step.
Disclosure: Long $BPAX
$BPAX have a close catalyst, BioSante will present at three healthcare conferences in September. The three presentations are as follows:
Wednesday, September 7, 2011 at 9:45 am EDT at the Stifel Nicolaus Healthcare Conference in Boston;
Monday, September 12, 2011 at 1:35 pm EDT at the Rodman & Renshaw Healthcare Conference in New York;
And on September 27, 2011 at 2:00 pm EDT at the JMP Securities Healthcare Conference in New York.
BioSante will provide a company overview at each conference, as well as an update on the LibiGel® (testosterone gel) Phase III clinical development program and planned NDA "new drug application" submission.
Tuesday, September 6, 2011
$BPAX Technical Analysis: Trading in an ascending triangle
$BPAX after the big drop following the markets meltdown, finding support @ the 200MA area.
In the past 4 weeks, we can see a price consolidation creating an Ascending Triangle ,we can see on the daily chart bellow higher lows, as the traders are paying more for the same shares.
The $2.50 horizontal line use as a support / resistance since 2009, and this days we can see a fight around this point too.
The indicators showing a strength as both the MACD & the RSI-14 with a Positive Divergence that gave me the confidence that the direction is upward, is might trade inside the triangle, but breaking up the upper line (white line) will give us a big move upward and we can calculate it as the side of the triangle that will give us a $0.80 at least...so the technical target is $3.60 at least....
Today. the price breakup the $2.50, but we need to see the price after the market closing, the next point is breaking up the upper line around $2.80.
$BPAX have a close catalyst, BioSante will present at three healthcare conferences in September. The three presentations are as follows:
Wednesday, September 7, 2011 at 9:45 am EDT at the Stifel Nicolaus Healthcare Conference in Boston;
Monday, September 12, 2011 at 1:35 pm EDT at the Rodman & Renshaw Healthcare Conference in New York;
And on September 27, 2011 at 2:00 pm EDT at the JMP Securities Healthcare Conference in New York.
BioSante will provide a company overview at each conference, as well as an update on the LibiGel® (testosterone gel) Phase III clinical development program and planned NDA "new drug application" submission.
Disclosure: Long $BPAX
Daily Chart
In the past 4 weeks, we can see a price consolidation creating an Ascending Triangle ,we can see on the daily chart bellow higher lows, as the traders are paying more for the same shares.
The $2.50 horizontal line use as a support / resistance since 2009, and this days we can see a fight around this point too.
The indicators showing a strength as both the MACD & the RSI-14 with a Positive Divergence that gave me the confidence that the direction is upward, is might trade inside the triangle, but breaking up the upper line (white line) will give us a big move upward and we can calculate it as the side of the triangle that will give us a $0.80 at least...so the technical target is $3.60 at least....
Today. the price breakup the $2.50, but we need to see the price after the market closing, the next point is breaking up the upper line around $2.80.
$BPAX have a close catalyst, BioSante will present at three healthcare conferences in September. The three presentations are as follows:
Wednesday, September 7, 2011 at 9:45 am EDT at the Stifel Nicolaus Healthcare Conference in Boston;
Monday, September 12, 2011 at 1:35 pm EDT at the Rodman & Renshaw Healthcare Conference in New York;
And on September 27, 2011 at 2:00 pm EDT at the JMP Securities Healthcare Conference in New York.
BioSante will provide a company overview at each conference, as well as an update on the LibiGel® (testosterone gel) Phase III clinical development program and planned NDA "new drug application" submission.
Disclosure: Long $BPAX
Daily Chart
Wednesday, August 31, 2011
$CLSN Technical Analysis:RunUp with lots of resistance points
$CLSN had a good support level around the $2.71 point as my last analysis and start moving upward along with the markets correction, had a pause @ $3.10 point and the buyers push the price up again breaking up the important resistance point @ $3.50 but with a low volume !!!!
This area is very complicated, full of support & resistance points, the main resistance now is the uptrend line that used as a support for a long period, and these days change his role and might used as a resistance line (the green line) we can see that the price break out the line, but retreat and close below the line. We might see a correction and recheck the $3.50 line that supposed to use as a support line this time, one more point is the price went out from the upper Bollinger Band, and might get some pressure to get back inside the bands, in the other side, breaking out the uptrend line will find another resistance point @ $3.87
Keep watching this important levels, and the markets direction will help CLSN to follow too.
Disclosure: Long $CLSN
Daily Chart
This area is very complicated, full of support & resistance points, the main resistance now is the uptrend line that used as a support for a long period, and these days change his role and might used as a resistance line (the green line) we can see that the price break out the line, but retreat and close below the line. We might see a correction and recheck the $3.50 line that supposed to use as a support line this time, one more point is the price went out from the upper Bollinger Band, and might get some pressure to get back inside the bands, in the other side, breaking out the uptrend line will find another resistance point @ $3.87
Keep watching this important levels, and the markets direction will help CLSN to follow too.
Disclosure: Long $CLSN
Daily Chart
Sunday, August 28, 2011
$AEZS Technical Analysis, Positive Divergence
$AEZS suffered from the economic meltdown as the other biotech stocks, and we saw a big drop from the $2.20 area to find a support @ the $1.44 horizontal support line as I mentioned a week ago, we saw a bullish Long Legged Doji as a reversal candlestick, followed by a bullish hammer to confirm the reversal pattern, and nice run-up from $1.44 to $1.95 in a 5 trading days.
After this run-up we can see a price consolidation crating a Pennant...
Pennant is a short-term continuation pattern that mark a small consolidation before the previous move resumes. These patterns are usually preceded by a sharp advance or decline with heavy volume, and mark a mid-point of the move.
If we look at the indicators and the momentum, we can see a Positive Divergence giving us an indication about the direction...Breaking up the pennant will give us a great run-up toward the first resistant point @ $2.19
First of all, we have to watch the markets carefully, as the direction of the biotech sector effected from the global sentiment, and it will keep effecting $AEZS too, the coming days are very important for the markets as we saw a support in the SP-500 and a positive week, the biotech stocks will follow the markets these days, but they will have larger recovery as they suffered from a big drop, and the companies with the closest catalyst will enjoy from a great run-up if the markets find the real support and start moving side-way or upward.
AEZS have a great potential, with a late stage as the AEZS-130 (orally active growth hormone stimulating agent) On 7/26/11, announced completion of P3 trial under SPA as oral diagnostic test for adult growth hormone deficiency, preparing for pre-NDA mtg w/ FDA to support planned NDA filing in the second half of 2011.
Perifosine (KRX-0401) (PI3K/Akt pathway inhibitor anti-cancer agent) On 7/27/11, $KERX the North American licensee, completed enrollment in the ongoing pivotal P3 trial for metastatic colorectal cancer, FDA Fast Track status with results expected by late 2011-early 2012
Disclosure: Long $AEZS
Long Legged Doji shows that there is a great deal of confusion and indecision in the market. This particular pattern shows that the prices moved well above and below the day's opening level, however they finally closed virtually at the same level with the opening price. The end result is only a little change from the opening price despite the whole volatility and excitement during the day that clearly reflects that the market lost its sense of direction.
After this run-up we can see a price consolidation crating a Pennant...
Pennant is a short-term continuation pattern that mark a small consolidation before the previous move resumes. These patterns are usually preceded by a sharp advance or decline with heavy volume, and mark a mid-point of the move.
If we look at the indicators and the momentum, we can see a Positive Divergence giving us an indication about the direction...Breaking up the pennant will give us a great run-up toward the first resistant point @ $2.19
First of all, we have to watch the markets carefully, as the direction of the biotech sector effected from the global sentiment, and it will keep effecting $AEZS too, the coming days are very important for the markets as we saw a support in the SP-500 and a positive week, the biotech stocks will follow the markets these days, but they will have larger recovery as they suffered from a big drop, and the companies with the closest catalyst will enjoy from a great run-up if the markets find the real support and start moving side-way or upward.
AEZS have a great potential, with a late stage as the AEZS-130 (orally active growth hormone stimulating agent) On 7/26/11, announced completion of P3 trial under SPA as oral diagnostic test for adult growth hormone deficiency, preparing for pre-NDA mtg w/ FDA to support planned NDA filing in the second half of 2011.
Perifosine (KRX-0401) (PI3K/Akt pathway inhibitor anti-cancer agent) On 7/27/11, $KERX the North American licensee, completed enrollment in the ongoing pivotal P3 trial for metastatic colorectal cancer, FDA Fast Track status with results expected by late 2011-early 2012
Disclosure: Long $AEZS
Sunday, August 21, 2011
$AMRN Technical Analysis, The support is very close.
$AMRN well know to most of the Biotech traders after it's huge GAP & runup on April this year, it's the dream of every trader to wake up one day and your position almost doubled overnight, the top was on May 31st reaching $19.87, but it start trading in a falling mode from that date in a downtrend channel, the price formed a support zone @ $13.30 trading 2 weeks side way, but with the help from the markets troubles and the big sell-off, it breaks down the support line and fall down till it touched the lower channel line and the 200MA to bouncing back from it...we can see that the both lines used as a support in one side and as a resistance in the second side.
The last trading day on Friday, the price break down again the 200MA heading toward the support line @ $9.66, breaking this point will send it toward $8.82, but I believe that this support will hold, as we can see a positive divergence on the RSI14, and the last candle touch the lower line of the Bollinger Bands that "mostly" the price bounce back from it.
Monday is an important day for the direction, if the price keep the downtrend, we might see it touching again the downtrend channel line, in the other side the $9.66 point supposed to use as a support.
I opened a small position @ $10.10 looking for a bounce back, but if the markets keep it's downtrend, I will cut losses with a stop loss, a few points below the support line, it's a well know way of a technical traders, to start a partial position in a stock that you like to own, close to a support line, it's easy as you have a predefined stop loss, close to your buying point, and if the trend goes in your favor, you can add more in the uptrend, after the next technical correction.
Disclosure:Long AMRN
The last trading day on Friday, the price break down again the 200MA heading toward the support line @ $9.66, breaking this point will send it toward $8.82, but I believe that this support will hold, as we can see a positive divergence on the RSI14, and the last candle touch the lower line of the Bollinger Bands that "mostly" the price bounce back from it.
Monday is an important day for the direction, if the price keep the downtrend, we might see it touching again the downtrend channel line, in the other side the $9.66 point supposed to use as a support.
I opened a small position @ $10.10 looking for a bounce back, but if the markets keep it's downtrend, I will cut losses with a stop loss, a few points below the support line, it's a well know way of a technical traders, to start a partial position in a stock that you like to own, close to a support line, it's easy as you have a predefined stop loss, close to your buying point, and if the trend goes in your favor, you can add more in the uptrend, after the next technical correction.
Disclosure:Long AMRN
Thursday, August 18, 2011
$SPX Tachnical Analysis, Looking for support
Weekly Chart
I will start with the long term and looking @ the weekly chart, we can see the last downtrend is a technical correction for the long period uptrend that started on March 2009 from 666.79 and finished with the highest level on May 2011 touching 1370.58....
The drop breakdown a long term uptrend line (purple) with a long candle and huge volume, the correction found a support @ the Fibonacci 38.2 level, bouncing back from that point, this week started well but the trend after Thursday big drop create another red candle, and This Friday close, will effect the whole week, is the price going down further to recheck the last support and create a double bottom??
Now for the short term we are moving to the daily chart trying to figure the support points & the indicators directions.
Daily Chart
The indicators are deep in the oversold zone, and need to recover, but they can stay there for longer period, I don't like the MACD that didn't cross up the moving average while the price went up sharply, that indicate that there is no momentum for the last uptrend, also the volume decrease in the uptrend days and increase in the down trend days..
Most of you, that read my tweets about the $SPX noticed that I mentioned several times, It's rare to see a V shape correction in the markets after a big drop, the W shape is more common and the price will drop back to recheck the last support (or close to it) to create a double bottom ....
So if the price check back the 1100 point and bounce back, it will be a bullish sign, but breaking it down and closing under that point will give us the sign that the bears won the battle and we are heading to the Fibo-50.0 point around 1020 as you can see it with the yellow lines in the weekly chart.
I will be looking for the close on Friday, maybe it will give us a clue about the overall direction, in these markets with a lots of volatility, we have to trade wisely and not invest, as it's hard to predict the direction and need to watch the charts of the markets daily and try to ride on the intraday volatility and work on 60min & 15min to get a short life trade.
I will start with the long term and looking @ the weekly chart, we can see the last downtrend is a technical correction for the long period uptrend that started on March 2009 from 666.79 and finished with the highest level on May 2011 touching 1370.58....
The drop breakdown a long term uptrend line (purple) with a long candle and huge volume, the correction found a support @ the Fibonacci 38.2 level, bouncing back from that point, this week started well but the trend after Thursday big drop create another red candle, and This Friday close, will effect the whole week, is the price going down further to recheck the last support and create a double bottom??
Now for the short term we are moving to the daily chart trying to figure the support points & the indicators directions.
Daily Chart
The indicators are deep in the oversold zone, and need to recover, but they can stay there for longer period, I don't like the MACD that didn't cross up the moving average while the price went up sharply, that indicate that there is no momentum for the last uptrend, also the volume decrease in the uptrend days and increase in the down trend days..
Most of you, that read my tweets about the $SPX noticed that I mentioned several times, It's rare to see a V shape correction in the markets after a big drop, the W shape is more common and the price will drop back to recheck the last support (or close to it) to create a double bottom ....
So if the price check back the 1100 point and bounce back, it will be a bullish sign, but breaking it down and closing under that point will give us the sign that the bears won the battle and we are heading to the Fibo-50.0 point around 1020 as you can see it with the yellow lines in the weekly chart.
I will be looking for the close on Friday, maybe it will give us a clue about the overall direction, in these markets with a lots of volatility, we have to trade wisely and not invest, as it's hard to predict the direction and need to watch the charts of the markets daily and try to ride on the intraday volatility and work on 60min & 15min to get a short life trade.
Wednesday, August 17, 2011
$ALXA Technical Analysis: waiting for the FDA reply
$ALXA started it's run-up early on Dec. 2010 from $0.90 to double and reach the $1.80 point on 03/30 and the highest point $1.91 on 06/30 enjoying the hype of the resubmission.
During the run-up stage we can see on the chart, the technical correction twice that had a good support of the uptrend line (purple line) .
The last markets sell-off hit $ALXA too...but it dropped very fast breaking down all the support levels...we can see that the strong suppprt @ $1.52 with the uptrend line & the horizontal line pause the drop for 5 trading days...but the power of the markets and the crowd over selling all the biotech sector hit $ALXA again to break down the $1.52 support, following with the next one @ $1.38 and the downtren line that supposed to use as a support , but all failed till the last one @ $1.15....
Do you think that this support will hold...IMHO the situation in the US markets have a big effect on this stock, and the Catalyst of the resubmission was on Aug. 05th, so $ALXA expecting the FDA reply on 14 days that lead us to the Aug. 19th, if the FDA accept the resubmission, they will give them the classification of the review, and most likely it will be Class-II with 6 months review period.
In a usual markets condition, the price would be in the highest levels, anticipating the FDA decision...and usually we see a "sell the news" action and profit taking, but in days & markets oversold like this days, there will be no sell-off as most the short term traders are out of this trade, we might see a spike toward the next resistant line around $1.38, but then it will fade as the 6 months is a long period for the ST traders, it might be a good point for the LT investors, but 6 months with no other catalyst or action during this period will put a pressure on the price, it will be a very good to check it back 3 months ahead of the new PDUFA date.
Many traders have the same issue with $PLX, as they are waiting for the FDA reply with Class-II review most likely too...but with $PLX it's different situation, as for Protalix there is 2 other catalyst waiting very close during the Q4, as they will have the European & the Brazilian decision during the end of Oct - Nov 2011, so the price this days backed in the Class-II decision and looking for the next catalyst.
Disclosure: No Position in ALXA
During the run-up stage we can see on the chart, the technical correction twice that had a good support of the uptrend line (purple line) .
The last markets sell-off hit $ALXA too...but it dropped very fast breaking down all the support levels...we can see that the strong suppprt @ $1.52 with the uptrend line & the horizontal line pause the drop for 5 trading days...but the power of the markets and the crowd over selling all the biotech sector hit $ALXA again to break down the $1.52 support, following with the next one @ $1.38 and the downtren line that supposed to use as a support , but all failed till the last one @ $1.15....
Do you think that this support will hold...IMHO the situation in the US markets have a big effect on this stock, and the Catalyst of the resubmission was on Aug. 05th, so $ALXA expecting the FDA reply on 14 days that lead us to the Aug. 19th, if the FDA accept the resubmission, they will give them the classification of the review, and most likely it will be Class-II with 6 months review period.
In a usual markets condition, the price would be in the highest levels, anticipating the FDA decision...and usually we see a "sell the news" action and profit taking, but in days & markets oversold like this days, there will be no sell-off as most the short term traders are out of this trade, we might see a spike toward the next resistant line around $1.38, but then it will fade as the 6 months is a long period for the ST traders, it might be a good point for the LT investors, but 6 months with no other catalyst or action during this period will put a pressure on the price, it will be a very good to check it back 3 months ahead of the new PDUFA date.
Many traders have the same issue with $PLX, as they are waiting for the FDA reply with Class-II review most likely too...but with $PLX it's different situation, as for Protalix there is 2 other catalyst waiting very close during the Q4, as they will have the European & the Brazilian decision during the end of Oct - Nov 2011, so the price this days backed in the Class-II decision and looking for the next catalyst.
Disclosure: No Position in ALXA
Monday, August 15, 2011
$CLSN Technical Analysis: Found the support, now the reversal starts
$CLSN enjoyed from a lots of hype and love from the Biotech investors lately, but also suffered from a big drop the same as all the biotech sector after the big sell-off in the US markets, despite the good PR about the completion of enrollment of 600 patients.
The big drop find a good support @ the weekly horizontal line $2.71 touching and breaking out the line 4 times but always close above it, giving us the feeling that this point is a good support, beside the 200MA in the area using as resistance and support.
The RSI & the Stochastics are deep in the oversold zone changing the direction of the lines and preparing for a runup.
I think, we can see the price in an uptrend as more as the markets do so, The first point that used as a resistance several times is $3.10 and did so today. we have to see the break out that point with a larg volume too to ensure the trend and direction.
It's important to feel the markets move, as if the markets turn positive, CLSN will have a wild run back to the last heights...as the investors will be back to the market and to the loved and preferred Bio stocks.
Disclosure: Long CLSN
The big drop find a good support @ the weekly horizontal line $2.71 touching and breaking out the line 4 times but always close above it, giving us the feeling that this point is a good support, beside the 200MA in the area using as resistance and support.
The RSI & the Stochastics are deep in the oversold zone changing the direction of the lines and preparing for a runup.
I think, we can see the price in an uptrend as more as the markets do so, The first point that used as a resistance several times is $3.10 and did so today. we have to see the break out that point with a larg volume too to ensure the trend and direction.
It's important to feel the markets move, as if the markets turn positive, CLSN will have a wild run back to the last heights...as the investors will be back to the market and to the loved and preferred Bio stocks.
Disclosure: Long CLSN
PROTALIX Submits Reply to FDA CRL as my evalutaion
In the first article that I wrote about PROTALIX early July I stated:
"I am confident that PROTALIX is close to the resubmission. I believe that the Company will be able to address all of the requests the FDA had asked for in their complete response approximately near the end of July until mid-August.
Today August 01st PROTALIX Submits Reply to FDA Complete Response Letter for Taliglucerase Alfa and Reports Top-Line Results from the Company's Switchover Trial.
The Company's submission addresses the issues identified by the FDA in the Complete Response Letter, including the request for clinical data from the Company's switchover trial and long-term extension trial, and additional information relating to chemistry, manufacturing and controls (CMC).
Data from all twenty six adult patients enrolled in the Company's switchover trial of patients switched from Cerezyme® to taliglucerase alfa over the nine-month period, were included in the submission. The data supports the efficacy and safety data package showing that patients can be switched from imiglucerase (Cerezyme®) to taliglucerase alfa. One patient experienced a hypersensitivity reaction. The efficacy data demonstrates that mean hemoglobin and platelet count, spleen volume and liver volume remained stable. Patients enrolled in the trial were switched from imiglucerase (doses ranging from around 10-60 U/kg every other week) to an equivalent dose using the same number of units of taliglucerase alfa.
The submission also included data from treatment naïve patients who completed the Company's pivotal Phase III trial and have continued to receive taliglucerase alfa for over 24 months in the Company's blinded long-term extension trial. These patients continued to show an improvement in efficacy and the drug was safe and well tolerated. Furthermore, those patients who were followed specifically for their bone parameters using Quantitative Chemical Shift Imaging
(QCSI) MRI continued to show bone marrow improvement over time. Detailed data from the Company's switchover trial and long-term extension trial will be presented at upcoming medical meetings.
Regarding CMC, Protalix submitted further analyses and modifications of analyses previously submitted to the FDA to address their questions raised with regard to testing specifications and assay validation.
After the resubmission completed, PROTALIX expects the FDA to provide an updated Prescription Drug User Fee Act (PDUFA) target action date within 14 days.
The FDA review team and division director will determine the classification of the
response, and a letter will be issued to the applicant acknowledging receipt of the
resubmission within 14 calendar days stating the classification of and the due date for action on the resubmission.
Class I is a two month review from the date of submissionClass II is a six month review from the date of submission
I anticipate that PROTALIX will get a Class II resubmission according to the data that was requested by FDA. If they receive a Class I resubmission, it may benefit shareholders significantly since there is less time to discount the event of possible approval.
I received so many emails and direct messages asking me, if PLX got Class-II, is it going to suffer a sell off too? The same as we usually see in the Biotech land these days.
I think that there will be no sell off, or a small action of profit taking, as most of the investors following PLX are a long term investors, and most of the biotech retail investors that usually following the catalyst run up, leave after it's done and jump to the second play, most of them already left the short term trade during the market volatility and shake out waiting for the debt ceiling issue to be solved.
As most of these investors already know that most likely Protalix will get Class-II review, so will be no surprise at all, and only the investors that believe on Protalix's potential will keep holding, knowing that beside the FDA review there is the European and the Brazilian review too, both are a real catalyst by themselves.
I will keep holding PLX through the decision, and wait till the PDUFA date, Technically we can see a solid support around $6.20, and if there will be a profit taking, I don't think that the price will break out this support level, in the other side, we know that Protalix can run up very fast to the $10 - $11 levels, as already did last year, and the resubmission is much safer than the first PDUFA and PLX most likely get the FDA approval.
Disclosure: Long PLX
"I am confident that PROTALIX is close to the resubmission. I believe that the Company will be able to address all of the requests the FDA had asked for in their complete response approximately near the end of July until mid-August.
Today August 01st PROTALIX Submits Reply to FDA Complete Response Letter for Taliglucerase Alfa and Reports Top-Line Results from the Company's Switchover Trial.
The Company's submission addresses the issues identified by the FDA in the Complete Response Letter, including the request for clinical data from the Company's switchover trial and long-term extension trial, and additional information relating to chemistry, manufacturing and controls (CMC).
Data from all twenty six adult patients enrolled in the Company's switchover trial of patients switched from Cerezyme® to taliglucerase alfa over the nine-month period, were included in the submission. The data supports the efficacy and safety data package showing that patients can be switched from imiglucerase (Cerezyme®) to taliglucerase alfa. One patient experienced a hypersensitivity reaction. The efficacy data demonstrates that mean hemoglobin and platelet count, spleen volume and liver volume remained stable. Patients enrolled in the trial were switched from imiglucerase (doses ranging from around 10-60 U/kg every other week) to an equivalent dose using the same number of units of taliglucerase alfa.
The submission also included data from treatment naïve patients who completed the Company's pivotal Phase III trial and have continued to receive taliglucerase alfa for over 24 months in the Company's blinded long-term extension trial. These patients continued to show an improvement in efficacy and the drug was safe and well tolerated. Furthermore, those patients who were followed specifically for their bone parameters using Quantitative Chemical Shift Imaging
(QCSI) MRI continued to show bone marrow improvement over time. Detailed data from the Company's switchover trial and long-term extension trial will be presented at upcoming medical meetings.
Regarding CMC, Protalix submitted further analyses and modifications of analyses previously submitted to the FDA to address their questions raised with regard to testing specifications and assay validation.
After the resubmission completed, PROTALIX expects the FDA to provide an updated Prescription Drug User Fee Act (PDUFA) target action date within 14 days.
The FDA review team and division director will determine the classification of the
response, and a letter will be issued to the applicant acknowledging receipt of the
resubmission within 14 calendar days stating the classification of and the due date for action on the resubmission.
Class I is a two month review from the date of submissionClass II is a six month review from the date of submission
I anticipate that PROTALIX will get a Class II resubmission according to the data that was requested by FDA. If they receive a Class I resubmission, it may benefit shareholders significantly since there is less time to discount the event of possible approval.
I received so many emails and direct messages asking me, if PLX got Class-II, is it going to suffer a sell off too? The same as we usually see in the Biotech land these days.
I think that there will be no sell off, or a small action of profit taking, as most of the investors following PLX are a long term investors, and most of the biotech retail investors that usually following the catalyst run up, leave after it's done and jump to the second play, most of them already left the short term trade during the market volatility and shake out waiting for the debt ceiling issue to be solved.
As most of these investors already know that most likely Protalix will get Class-II review, so will be no surprise at all, and only the investors that believe on Protalix's potential will keep holding, knowing that beside the FDA review there is the European and the Brazilian review too, both are a real catalyst by themselves.
I will keep holding PLX through the decision, and wait till the PDUFA date, Technically we can see a solid support around $6.20, and if there will be a profit taking, I don't think that the price will break out this support level, in the other side, we know that Protalix can run up very fast to the $10 - $11 levels, as already did last year, and the resubmission is much safer than the first PDUFA and PLX most likely get the FDA approval.
Disclosure: Long PLX
“While ALXA is running up in anticipation of an NDA filing scheduled in July, Protalix has been preparing to submit a complete response regarding the company’s NDA for Taliglucerase Alfa which may surprise the biotech trading community...”
“While ALXA is running up in anticipation of an NDA filing scheduled in July, Protalix has been preparing to submit a complete response regarding the company’s NDA for Taliglucerase Alfa which may surprise the biotech trading community...”
Author: Joe Gantoss
Protalix Biotherapeutics Inc. (PLX) is a biotech company focused on the development and commercialization of recombinant therapeutic proteins based on their proprietary ProCellEx(TM) protein expression system. By leveraging the ProCellEx platform, Protalix has created a strong pipeline of next generation biologics. The company’s initial focus has been on complex therapeutic proteins which include treatments for rare genetic disorders such as Gaucher disease and Fabry disease. Their lead product candidate, Taliglucerase Alfa, is an enzyme intended for the treatment of Gaucher disease.
Gaucher disease is a chronic, progressive, inherited genetic disorder caused by insufficient levels of a particular enzyme, glucocerebrosidase (GCD). As a result, fatty acids accumulate in certain cells in the body and can cause mild to severe symptoms. Patients can experience anemia and low platelet levels, yet such accumulation can even cause organ damage to areas such as the liver and spleen. The current treatments available in the market are enzyme replacement therapies, such as Genzyme’s Cerezyme (also known as Imiglucerase).
Protalix’s Journey
In July 2007, Protalix reached an agreement with the FDA on the final design of the pivotal phase III clinical trial of Taliglucerase Alfa by way of the special protocol assessment (SPA) process. Protalix completed the phase III clinical trial of Taliglucerase Alfa for the treatment of Gaucher disease in September 2009, and, on October 15, 2009, the Company announced positive top-line results.
Protalix filed a New Drug Application (NDA) for Taliglucerase Alfa on December 9, 2009, and on January 2010, the FDA requested additional data regarding the chemistry, manufacturing and controls (CMC) section of the NDA submission. Protalix provided the requested data to the FDA in April 2010, and on July 2010, the Company received notification from the FDA that they accepted Protalix’s filing and assigned a Prescription Drug User Fee Act (PDUFA) goal date on February 25, 2011.
In November 2010, Protalix submitted a marketing application for Taliglucerase Alfa to the Israeli Ministry of Health (MOH), and Pfizer, Protalix’s commercial partner, submitted a Marketing Authorization Application (MAA) to the European Medicines Agency (EMA). Protalix also filed marketing applications in Brazil and Australia. Let us keep this fact in mind as we continue on recollecting the data available to us.
Many believed that Taliglucerase Alfa had a shot on obtaining FDA approval (one prime example was Oppenheimer’s equity research on PLX), but on February 25, 2011, the FDA issued a Complete Response Letter (CRL) regarding the company’s NDA for Taliglucerase Alfa. A CRL is issued by the FDA's Center for Drug Evaluation and Research when the review of a file is completed and questions remain that precludes the approval of the NDA in its current form. The main questions raised by the FDA regarding the NDA related to the clinical and CMC sections. In the clinical section of the CRL, the FDA requested additional data from the ongoing switchover trial and the long-term extension trial. At the time the NDA was submitted, full data from these trials were not available. In the CMC section of the CRL, the FDA requested information regarding testing specifications and assay validation. The FDA did not however request additional clinical studies in the CRL.
One thing to note is that the marketing applications filed in the European Union, Brazil and Australia include certain data now being requested by the FDA in the CRL (as stated on Protalix’s most recent 10-Q on Pg. 9). These applications (the marketing applications) were submitted after filing the NDA, during which Protalix had collected additional data from these ongoing trials.
Manufacturing Audits
On February 2010, the Israeli MOH successfully completed a good manufacturing practices (GMP) audit of the manufacturing facilities in Carmiel, Israel. The audit was performed as part of the Israeli MOH's evaluation of Protalix manufacturing process for Taliglucerase Alfa.
On February 20, 2011, Protalix received a letter from the FDA notifying that the FDA had completed its review of the Establishment Inspection Report in connection with the FDA's inspection of Protalix facility in Carmiel, Israel, and that the FDA had classified the facility as acceptable.
On June 16, 2011 Protalix announced the successful GMP manufacturing audit by Brazil’s National Health Surveillance Agency.
Protalix Completes Review Meeting with FDA, NDA Resubmission in Near-Term
Protalix’s management has been preparing to submit a complete response, given Pfizer’s co-operation, who has worldwide commercial rights to the Taliglucerase Alfa (except in Israel, where Protalix retains the right to commercialize Taliglucerase Alfa). The first step in the process was a meeting scheduled with the FDA in May 2011. At that meeting, Protalix received certain clarifications regarding the CRL. Dr. Aviezer, Protalix’s President and CEO, informed me that during this face-to-face meeting with the FDA, the agency did not raise any new issues. The discussion at the meeting clarified the information requested by the FDA. Protalix believes they can provide the additional data requested and are working to ensure the response is thorough and complete.
One month after Protalix received the CRL, I had the opportunity to meet with Dr. David Aviezer at Protalix’s headquarters in Carmiel, in which also in attendance was a small group of investors. The objective of the meeting was to better understand the issues outlined in the CRL and how Protalix’s management will properly address to those certain issues. The group also had the opportunity to tour the manufacturing plant. I left the meeting with a positive impression: Confident that the Protalix team, along with their Pfizer partners, have been working diligently to collect the additional data and generate the information requested. The company plans to submit a complete response in a couple of months, as stated on their 10-Q on page 10.
Recently, after meeting with the company’s Investor Relations firm, the Trout Group (NYC), and speaking again with Dr. David Aviezer, I am confident that Protalix is close to the resubmission. I believe that the Company will be able to address all of the requests the FDA had asked for in their complete response approximately near the end of July until mid-August.
After the resubmission, the FDA will notify the Company within 14 days if they accepted the NDA resubmission and assign a Class I or Class II review:
· Class I is a two month review from the date of submission
· Class II is a six month review from the date of submission
I anticipate that Protalix will get a Class II resubmission according to the data that was requested by FDA. If they receive a Class I resubmission, it may benefit shareholders significantly since there is less time to discount the event of possible approval.
If Taliglucerase Alfa is granted FDA approval, then this will trigger additional regulatory milestone payments of up to $50 million Pfizer obligated to pay to Protalix, as part of their exclusive license and supply agreement.
Additional Catalysts in the Near Future
While Protalix is planning to prepare for upcoming FDA action, recall that Protalix has other programs in progress for Taliglucerase Alfa outside of the U.S.
Brazil
Approval in Brazil may precede the FDA approval. The Brazilian approval process through The National Health Surveillance Agency (in Portuguese, Agência Nacional de Vigilância Sanitária, ANVISA) is not connected in any way to the FDA’s approval process and hence Protalix could gain approval before year end in this country. Approval in Brazil could trigger a long-term supply agreement that contemplates transferring certain components of the manufacturing technology to the Ministry of Health of Brazil. I do not expect a long-term supply agreement to be signed before Protalix receives marketing approval of Taliglucerase Alfa from the ANVISA.
Note that a $30M supply agreement has already been executed in Brazil. On August 10, 2010, Pfizer entered into a $30 million short-term supply agreement with the Ministry of Health of Brazil pursuant to which Protalix and Pfizer provided Taliglucerase Alfa to the Ministry of Health of Brazil for the treatment of patients with Gaucher disease. During the first quarter of 2011, Protalix and Pfizer supplied the remaining products deliverable under the short-term supply agreement. The revenue generated from the Ministry of Health of Brazil was recorded by Pfizer, and Protalix recorded its share of the revenue in accordance with the terms and conditions of the Pfizer agreement (Pfizer 60% and Protalix 40%)
European Union
In November 2010, Pfizer submitted a Marketing Authorization Application (MAA) to the European Medicines Agency (EMA). Pfizer received the 120 day questions in April 2011, with a GMP audit planned for 3Q 2011. The EMA decision date will approximately take roughly a year from the submission date, so Protalix expects a European decision around year end in 2011. Approval in Europe will trigger another milestone payment from Pfizer to Protalix as stated on their license and supply agreement.
The rest of the world
To date, given that marketing applications for Taliglucerase Alfa have been submitted in the US, European Union, Brazil, and Israel, recall that Pfizer also submitted a marketing application for Taliglucerase Alfa in Australia in May 2011. The Australian approval process through The Advisory Committee on Prescription Medicine (ACPM) formally known as the Australian Drug Evaluation Committee (ADEC) approximately endures a year’s worth of time from the submission date to the ACPM decision date. Therefore, Protalix expects an Australian decision around the second quarter of 2012.
New Drugs on the Horizon
In addition to Taliglucerase Alfa, Protalix is developing an innovative product pipeline using their patented ProCellEx protein expression system. The pipeline currently includes the following:
PRX-102
This product is a therapeutic protein candidate that is biologically and chemically modified to improve upon the current treatments for Fabry Disease, a rare, genetic lysosomal disorder. The Company is in advanced R&D development, which includes product characterization and determination of release specifications. Protalix has performed proof of concept studies in a “Fabry mice” knock out model. The company held a pre-IND meeting with the FDA in December of 2010. This year, Protalix will conduct more toxicology studies and plans to submit an IND to the FDA.
PRX-105
This product is a plant cell expressed pegylated recombinant acetylcholinesterase (AChE) which is intended for biodefense and CNS. In March 2010, Protalix initiated a preliminary phase I clinical trial of PRX-105 which was completed in June 2010. Protalix is currently preparing for further efficacy trials of this product candidate in large animal models.
PRX-106: Anti-TNF (Enbrel Biosimilar)
This product is a plant cell expressed recombinant anti-TNF fusion protein made from the soluble form of the human TNF receptor (TNFR) and an antibody portion. The amino acid sequence is identical to ENBREL. PRX-106 is intended for the treatment of certain auto immune diseases such as rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing, spondylitis, psoriatic arthritis and plaque psoriasis.
Financial Status
Their last proposed public offering of common stock was approximately 4.9% of their total shares outstanding which was in effect after PLX received the CRL. Management now has sufficient cash ($50.23M) to comfortably withstand the FDA review cycle for Taliglucerase Alfa.
Share Price (closing 07/08) $6.68
52-Week High $10.60
52-Week Low $5.74
Average Daily Volume (sh) 211,111
Shares Outstanding (mil) 85.577
Float (Mil) 64.161
Market Capitalization ($mil) $529.73
Institutional Ownership (%) 21.1
Insider Ownership (%) 9.66
Total Cash (Mil as 3/31/2011) $50.2
Short interest (mil Sh.) 2.71
Short interest Prior (mil Sh.) 2.80
Short % Decrease -3.11
Short Interest Ratio (days) 7.9
Technical Analysis
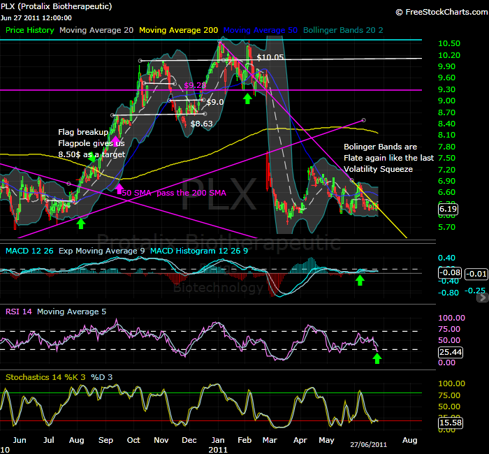
As shown below, given the price drop Protalix suffered after receiving the CRL from the FDA on February 25 2011, and how the price was testing the support region on March 18, 2011 (the dilution date) the same day of that drop, the price initiated a slow reversal followed up by a flat trend. The Bollinger Bands at this time began to compress to each other causing a sign of a volatility squeeze, the same volatility squeeze shown last year.
This volatility squeeze cause the price to move from the $6.5 level to the highs of $10.05 in two months, shortly accompanied with a small correction, then reaching to a new high of $10.60. The resubmission that is to be expected in the span of 2 months is believed to be the catalyst to jump start the new uptrend towards the new PDUFA date. I think, provided this recollection of data, we will see the same uptrend this year as well.
Disclosure: Long PLX
Author: Joe Gantoss
Protalix Biotherapeutics Inc. (PLX) is a biotech company focused on the development and commercialization of recombinant therapeutic proteins based on their proprietary ProCellEx(TM) protein expression system. By leveraging the ProCellEx platform, Protalix has created a strong pipeline of next generation biologics. The company’s initial focus has been on complex therapeutic proteins which include treatments for rare genetic disorders such as Gaucher disease and Fabry disease. Their lead product candidate, Taliglucerase Alfa, is an enzyme intended for the treatment of Gaucher disease.
Gaucher disease is a chronic, progressive, inherited genetic disorder caused by insufficient levels of a particular enzyme, glucocerebrosidase (GCD). As a result, fatty acids accumulate in certain cells in the body and can cause mild to severe symptoms. Patients can experience anemia and low platelet levels, yet such accumulation can even cause organ damage to areas such as the liver and spleen. The current treatments available in the market are enzyme replacement therapies, such as Genzyme’s Cerezyme (also known as Imiglucerase).
Protalix’s Journey
In July 2007, Protalix reached an agreement with the FDA on the final design of the pivotal phase III clinical trial of Taliglucerase Alfa by way of the special protocol assessment (SPA) process. Protalix completed the phase III clinical trial of Taliglucerase Alfa for the treatment of Gaucher disease in September 2009, and, on October 15, 2009, the Company announced positive top-line results.
Protalix filed a New Drug Application (NDA) for Taliglucerase Alfa on December 9, 2009, and on January 2010, the FDA requested additional data regarding the chemistry, manufacturing and controls (CMC) section of the NDA submission. Protalix provided the requested data to the FDA in April 2010, and on July 2010, the Company received notification from the FDA that they accepted Protalix’s filing and assigned a Prescription Drug User Fee Act (PDUFA) goal date on February 25, 2011.
In November 2010, Protalix submitted a marketing application for Taliglucerase Alfa to the Israeli Ministry of Health (MOH), and Pfizer, Protalix’s commercial partner, submitted a Marketing Authorization Application (MAA) to the European Medicines Agency (EMA). Protalix also filed marketing applications in Brazil and Australia. Let us keep this fact in mind as we continue on recollecting the data available to us.
Many believed that Taliglucerase Alfa had a shot on obtaining FDA approval (one prime example was Oppenheimer’s equity research on PLX), but on February 25, 2011, the FDA issued a Complete Response Letter (CRL) regarding the company’s NDA for Taliglucerase Alfa. A CRL is issued by the FDA's Center for Drug Evaluation and Research when the review of a file is completed and questions remain that precludes the approval of the NDA in its current form. The main questions raised by the FDA regarding the NDA related to the clinical and CMC sections. In the clinical section of the CRL, the FDA requested additional data from the ongoing switchover trial and the long-term extension trial. At the time the NDA was submitted, full data from these trials were not available. In the CMC section of the CRL, the FDA requested information regarding testing specifications and assay validation. The FDA did not however request additional clinical studies in the CRL.
One thing to note is that the marketing applications filed in the European Union, Brazil and Australia include certain data now being requested by the FDA in the CRL (as stated on Protalix’s most recent 10-Q on Pg. 9). These applications (the marketing applications) were submitted after filing the NDA, during which Protalix had collected additional data from these ongoing trials.
Manufacturing Audits
On February 2010, the Israeli MOH successfully completed a good manufacturing practices (GMP) audit of the manufacturing facilities in Carmiel, Israel. The audit was performed as part of the Israeli MOH's evaluation of Protalix manufacturing process for Taliglucerase Alfa.
On February 20, 2011, Protalix received a letter from the FDA notifying that the FDA had completed its review of the Establishment Inspection Report in connection with the FDA's inspection of Protalix facility in Carmiel, Israel, and that the FDA had classified the facility as acceptable.
On June 16, 2011 Protalix announced the successful GMP manufacturing audit by Brazil’s National Health Surveillance Agency.
Protalix Completes Review Meeting with FDA, NDA Resubmission in Near-Term
Protalix’s management has been preparing to submit a complete response, given Pfizer’s co-operation, who has worldwide commercial rights to the Taliglucerase Alfa (except in Israel, where Protalix retains the right to commercialize Taliglucerase Alfa). The first step in the process was a meeting scheduled with the FDA in May 2011. At that meeting, Protalix received certain clarifications regarding the CRL. Dr. Aviezer, Protalix’s President and CEO, informed me that during this face-to-face meeting with the FDA, the agency did not raise any new issues. The discussion at the meeting clarified the information requested by the FDA. Protalix believes they can provide the additional data requested and are working to ensure the response is thorough and complete.
One month after Protalix received the CRL, I had the opportunity to meet with Dr. David Aviezer at Protalix’s headquarters in Carmiel, in which also in attendance was a small group of investors. The objective of the meeting was to better understand the issues outlined in the CRL and how Protalix’s management will properly address to those certain issues. The group also had the opportunity to tour the manufacturing plant. I left the meeting with a positive impression: Confident that the Protalix team, along with their Pfizer partners, have been working diligently to collect the additional data and generate the information requested. The company plans to submit a complete response in a couple of months, as stated on their 10-Q on page 10.
Recently, after meeting with the company’s Investor Relations firm, the Trout Group (NYC), and speaking again with Dr. David Aviezer, I am confident that Protalix is close to the resubmission. I believe that the Company will be able to address all of the requests the FDA had asked for in their complete response approximately near the end of July until mid-August.
After the resubmission, the FDA will notify the Company within 14 days if they accepted the NDA resubmission and assign a Class I or Class II review:
· Class I is a two month review from the date of submission
· Class II is a six month review from the date of submission
I anticipate that Protalix will get a Class II resubmission according to the data that was requested by FDA. If they receive a Class I resubmission, it may benefit shareholders significantly since there is less time to discount the event of possible approval.
If Taliglucerase Alfa is granted FDA approval, then this will trigger additional regulatory milestone payments of up to $50 million Pfizer obligated to pay to Protalix, as part of their exclusive license and supply agreement.
Additional Catalysts in the Near Future
While Protalix is planning to prepare for upcoming FDA action, recall that Protalix has other programs in progress for Taliglucerase Alfa outside of the U.S.
Brazil
Approval in Brazil may precede the FDA approval. The Brazilian approval process through The National Health Surveillance Agency (in Portuguese, Agência Nacional de Vigilância Sanitária, ANVISA) is not connected in any way to the FDA’s approval process and hence Protalix could gain approval before year end in this country. Approval in Brazil could trigger a long-term supply agreement that contemplates transferring certain components of the manufacturing technology to the Ministry of Health of Brazil. I do not expect a long-term supply agreement to be signed before Protalix receives marketing approval of Taliglucerase Alfa from the ANVISA.
Note that a $30M supply agreement has already been executed in Brazil. On August 10, 2010, Pfizer entered into a $30 million short-term supply agreement with the Ministry of Health of Brazil pursuant to which Protalix and Pfizer provided Taliglucerase Alfa to the Ministry of Health of Brazil for the treatment of patients with Gaucher disease. During the first quarter of 2011, Protalix and Pfizer supplied the remaining products deliverable under the short-term supply agreement. The revenue generated from the Ministry of Health of Brazil was recorded by Pfizer, and Protalix recorded its share of the revenue in accordance with the terms and conditions of the Pfizer agreement (Pfizer 60% and Protalix 40%)
European Union
In November 2010, Pfizer submitted a Marketing Authorization Application (MAA) to the European Medicines Agency (EMA). Pfizer received the 120 day questions in April 2011, with a GMP audit planned for 3Q 2011. The EMA decision date will approximately take roughly a year from the submission date, so Protalix expects a European decision around year end in 2011. Approval in Europe will trigger another milestone payment from Pfizer to Protalix as stated on their license and supply agreement.
The rest of the world
To date, given that marketing applications for Taliglucerase Alfa have been submitted in the US, European Union, Brazil, and Israel, recall that Pfizer also submitted a marketing application for Taliglucerase Alfa in Australia in May 2011. The Australian approval process through The Advisory Committee on Prescription Medicine (ACPM) formally known as the Australian Drug Evaluation Committee (ADEC) approximately endures a year’s worth of time from the submission date to the ACPM decision date. Therefore, Protalix expects an Australian decision around the second quarter of 2012.
New Drugs on the Horizon
In addition to Taliglucerase Alfa, Protalix is developing an innovative product pipeline using their patented ProCellEx protein expression system. The pipeline currently includes the following:
PRX-102
This product is a therapeutic protein candidate that is biologically and chemically modified to improve upon the current treatments for Fabry Disease, a rare, genetic lysosomal disorder. The Company is in advanced R&D development, which includes product characterization and determination of release specifications. Protalix has performed proof of concept studies in a “Fabry mice” knock out model. The company held a pre-IND meeting with the FDA in December of 2010. This year, Protalix will conduct more toxicology studies and plans to submit an IND to the FDA.
PRX-105
This product is a plant cell expressed pegylated recombinant acetylcholinesterase (AChE) which is intended for biodefense and CNS. In March 2010, Protalix initiated a preliminary phase I clinical trial of PRX-105 which was completed in June 2010. Protalix is currently preparing for further efficacy trials of this product candidate in large animal models.
PRX-106: Anti-TNF (Enbrel Biosimilar)
This product is a plant cell expressed recombinant anti-TNF fusion protein made from the soluble form of the human TNF receptor (TNFR) and an antibody portion. The amino acid sequence is identical to ENBREL. PRX-106 is intended for the treatment of certain auto immune diseases such as rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing, spondylitis, psoriatic arthritis and plaque psoriasis.
Financial Status
Their last proposed public offering of common stock was approximately 4.9% of their total shares outstanding which was in effect after PLX received the CRL. Management now has sufficient cash ($50.23M) to comfortably withstand the FDA review cycle for Taliglucerase Alfa.
Share Price (closing 07/08) $6.68
52-Week High $10.60
52-Week Low $5.74
Average Daily Volume (sh) 211,111
Shares Outstanding (mil) 85.577
Float (Mil) 64.161
Market Capitalization ($mil) $529.73
Institutional Ownership (%) 21.1
Insider Ownership (%) 9.66
Total Cash (Mil as 3/31/2011) $50.2
Short interest (mil Sh.) 2.71
Short interest Prior (mil Sh.) 2.80
Short % Decrease -3.11
Short Interest Ratio (days) 7.9
Technical Analysis
As shown below, given the price drop Protalix suffered after receiving the CRL from the FDA on February 25 2011, and how the price was testing the support region on March 18, 2011 (the dilution date) the same day of that drop, the price initiated a slow reversal followed up by a flat trend. The Bollinger Bands at this time began to compress to each other causing a sign of a volatility squeeze, the same volatility squeeze shown last year.
This volatility squeeze cause the price to move from the $6.5 level to the highs of $10.05 in two months, shortly accompanied with a small correction, then reaching to a new high of $10.60. The resubmission that is to be expected in the span of 2 months is believed to be the catalyst to jump start the new uptrend towards the new PDUFA date. I think, provided this recollection of data, we will see the same uptrend this year as well.
Disclosure: Long PLX

Sunday, August 14, 2011
$BPAX Technical Analysis - Looking for direction.
$BPAX had a great run-up started @ $2.50 mid of June till it reached the high of $4.02 on 07/13 creating a "Dark Cloud Cover" a bearish pattern, giving the first sign that the technical correction is coming, followed by several doji's to insure the confusion of the investors about the direction.
The correction break down the first support level @ $3.20 level heading to the second & strong support around $2.50 that used several times in the past as a support & resistance point...and with the help of the markets bearish sentiment during the "Debt Ceiling" issue followed by the markets sell-off, $Bpax price dropped toward the $2.00 level finding the 200MA as a support level.
The price break down a LT (long term) uptrend line and the 200MA but bounce back creating a Bullish Hammer as a reversal pattern followed by 3 doji's and a long green candlestick last Friday checking the resistance line @ $2.50 but closing below the line, I believe that the trend will continue and breakup the $2.50 level heading towards the second resistance line @ $3.20
We can see that all the indicators are in the oversold zone, that gave us a hint for the direction as an uptrend is coming, but we have to give more weight to the "Markets Sentiment" as in this case the markets can put a pressure @ the biotech sector and dragging $Bpax with it for a new downtrend.
Keep watching closely.
Disclosure: Long Bpax
The correction break down the first support level @ $3.20 level heading to the second & strong support around $2.50 that used several times in the past as a support & resistance point...and with the help of the markets bearish sentiment during the "Debt Ceiling" issue followed by the markets sell-off, $Bpax price dropped toward the $2.00 level finding the 200MA as a support level.
The price break down a LT (long term) uptrend line and the 200MA but bounce back creating a Bullish Hammer as a reversal pattern followed by 3 doji's and a long green candlestick last Friday checking the resistance line @ $2.50 but closing below the line, I believe that the trend will continue and breakup the $2.50 level heading towards the second resistance line @ $3.20
We can see that all the indicators are in the oversold zone, that gave us a hint for the direction as an uptrend is coming, but we have to give more weight to the "Markets Sentiment" as in this case the markets can put a pressure @ the biotech sector and dragging $Bpax with it for a new downtrend.
Keep watching closely.
Disclosure: Long Bpax
Subscribe to:
Posts (Atom)